The ingredient that will transform the plastics industry:
Discover the benefits of Graphenemex graphene masterbatches as a nucleation agent
The plastics industry constantly demands new reinforcements or additives that allow the improvement of plastic materials, both for commercial and engineering use. In recent years, the use of graphene and its derivatives (graphene oxide, GO) has been promoted as new reinforcements for different polymer matrices.
Graphene is a nanomaterial (nanometric particle) that has extraordinary electrical, optical, thermal properties and high mechanical resistance. The properties of graphene are attributed to its structure in the form of two-dimensional (2D) sheets, formed by carbon atoms linked in a hexagonal manner and a thickness of one carbon atom.
The incorporation of graphene materials in polymers allows the development of polymeric compounds with greater mechanical resistance, greater impact resistance, resistance to UV radiation and greater thermal stability, among other properties. This allows obtaining better materials, with great potential and a wide range of applications for different sectors (automotive, aerospace, electronics or packaging).
In general when we talk about traditional polymeric compounds, they are materials that contain a quantity (~40%) of reinforcement in the polymeric matrix. In contrast, polymeric compounds with graphene (nanocomposites), graphene improves the properties of the polymer with the use of low concentrations (<2% weight), as reinforcement. Various investigations have shown that polymers functionalized with graphene materials provide improvements in mechanical, thermal, and electrical properties. For example in:
- Polypropylene / Graphene compounds, showed an increase in flexural modulus (30%) and an increase in impact resistance (40%) compared to other commercial composites.
- Polyethylene / Graphene compound, improves tensile strength (17%), flexural strength and rupture strength (66%).
- Polystyrene/graphene compounds, showed an increase in electrical conductivity at room temperature from 0.1 to 1 S/m.

In addition to what was mentioned above, it is important to indicate that graphene materials function as nucleation agents in semicrystalline polymers. One of the most important characteristics of semicrystalline polymers is the degree of crystallinity. Many properties are influenced by the degree of crystallinity of the polymers.
While crystallinity in metals and ceramics implies the arrangement or arrangement of atoms and ions, in polymers it implies the arrangement of molecules and, therefore, the complexity is greater. Polymer crystallinity can be thought of as the packing of molecular chains to produce an ordered atomic arrangement. Because polymer molecules are large and complex, they are often partially crystalline (semi-crystalline) with scattered crystalline regions within an amorphous material. In the amorphous region, disordered chains appear, a very common condition due to twists, folds and folds of the chains that prevent the ordering of each segment of each chain.
In general, few polymers have a sufficient structure to crystallize and even in these cases, it is never possible to achieve 100% crystalline structure and the degree of crystallization (Xc) must be determined, that is, the fraction of the polymer that presents a crystalline structure in relation to the total polymer, the rest will be amorphous.
The general tendency of the addition of nucleating agents in polymeric matrices is the acceleration or retardation of crystallization, changes in the size of the spherulites, changes in the morphology and in some cases changes in the crystal structure. If we focus on the effect of graphene materials on the crystallinity of polymers, we can summarize that; Graphene materials make it possible to control the size of spherulites (crystal growth) in polymeric compounds, which leads to controlling the crystalline zones, which are responsible for mechanical resistance, and the amorphous zones (associated with flexibility and elasticity). of the material). In addition to improving interfacial adhesion in polymer matrices with polar groups, such as nylon 6,6. On the other hand, another advantage of graphene materials as a nucleating agent in polymeric compounds is that the crystallization temperature (Tc) increases as the amount of graphene increases because the crystallization of the melt is promoted, that is, Less energy is needed to cool the molten polymer, saving time and energy.
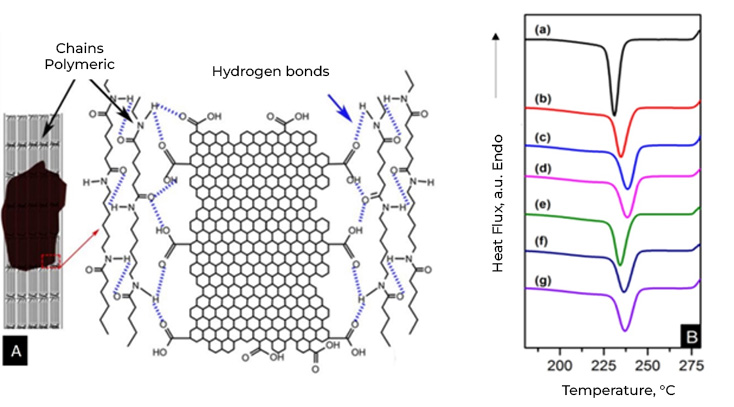
Currently Energeia – Graphenemex®, a leading Mexican company in Latin America in research and production of graphene materials for the development of applications at an industrial level, through its Graphenergy Masterbatch line, has developed and sells a wide range of masterbatches with graphene, based on various polymers, such as PP, HDPE, LDPE, PET and PA6. Our Masterbatches are granular materials that act as multifunctional reinforcements and effective nucleating agents.
References
- Gong, L., Yin, B., Li, L., & Yang, M. (2015). Nylon-6/Graphene composites modified through polymeric modification of graphene. Composites Part B: Engineering, 73, 49–56.
- Fabiola Navarro-Pardo, Gonzalo Martínez-Barrera, Ana Laura Martínez-Hernández, Víctor M. Castaño. Effects on the Thermo-Mechanical and Crystallinity Properties of Nylon 6,6 Electrospun Fibres Reinforced with One Dimensional (1D) and Two Dimensional (2D) Carbon. Materials 2013, 6.
- Zhang, F.; Peng, X.; Yan, W.; Peng, Z.; Shen, Y. Nonisothermal crystallization kinetics of in-situ nylon 6/graphene composites by differential scanning calorimetry. J. Polym. Sci. Part B. Polym. Phys. 2011, 49, 1381–1388.
- Yun, Y.S.; Bae, Y.H.; Kim, D.H.; Lee, J.Y.; Chin, I.J.; Jin, HJ. Reinforcing effects of adding alkylated graphene oxide to polypropylene. Carbon 2011, 49, 3553–3559.
- Cheng, S.; Chen, X.; Hsuan, Y.G.; Li, C.Y. Reduced graphene oxide induced polyethylene crystallization in solution and composites. Macromolecules 2012, 45, 993–1000.

