Graphene oxide:
a promising alternative in nanotechnology
Since graphene was isolated for the first time in 2004 by the Manchester group, this nanomaterial has proven to be the most revolutionary for the development of new applications at an industrial level.
Graphene has extraordinary electrical, optical, thermal properties and high mechanical resistance. The properties of graphene are attributed to its structure in the form of two-dimensional (2D) sheets, made up of hexagonally bonded carbon atoms and a thickness of one carbon atom.
Currently there are different methods of graphene production, these can be classified into two methods, according to their origin, the “bottom-up” method and the “top down” method. The “bottom-Up” method consists in the creation of graphene structures through building blocks (atoms, molecules), for example, by Chemical Vapor Deposition (CVD); and the “top down” method involves the production of graphene from the oxidation of graphite. Graphite is made up of sheets of graphene that are stacked on top of each other. The following diagram represents the process for obtaining graphene from the oxidation of graphite.
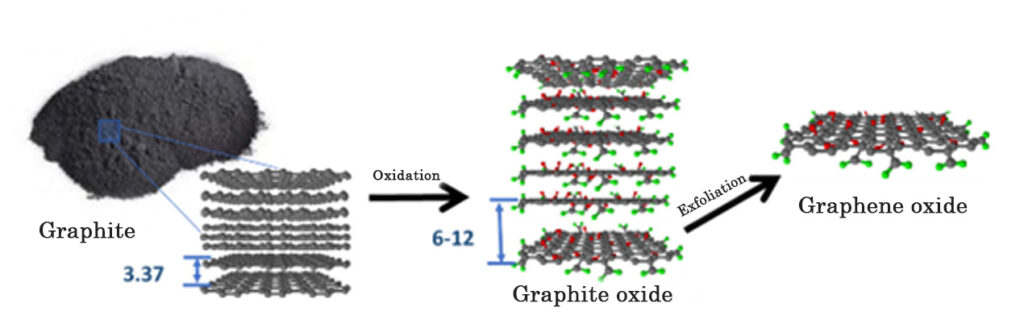
The graphite oxidation process begins with the addition of graphite in sulfuric acid (H2SO4), with constant mechanical stirring. Subsequently, potassium permanganate (KMnO4) is slowly added, producing a chemical reaction that allows the graphite (graphene sheets stacked on top of each other) to be chemically modified in its structure. When KMnO4 reacts with H2SO4, it forms manganese oxide VII (Mn2O7), which is a very selective oxidizing agent on double bond aromatic compounds, such as graphite. The oxidizing agent molecularly attacks the structure of each graphene sheet in the graphite, grafting oxygenated functional groups (with oxygen), such as epoxide groups (C-O-C) and hydroxyl groups (-OH), on each sheet, and carboxyl groups (-COOH, CO2H ) on the edges of each sheet, obtaining graphite oxide and graphene oxide (GO), see Figure 1.
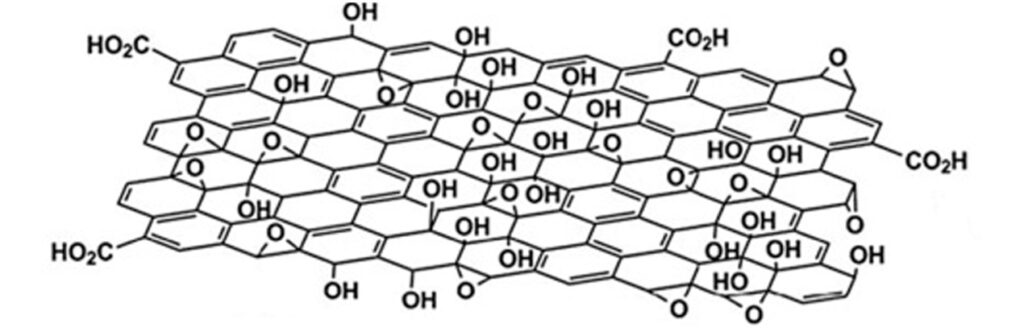
The incorporation of oxygenated functional groups allows a material such as graphite, which is highly hydrophobic (which repels water) and a good electrical conductor, to become graphite oxide and graphene oxide (GO), highly hydrophilic materials, that is, they mix and disperse easily with water (See Figure 2). GO is chemically similar to graphite oxide, but structurally differs in the arrangement and number of stacked sheets.
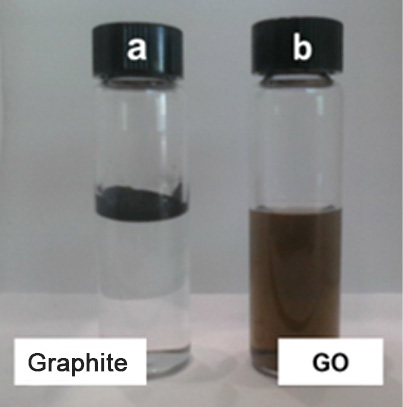
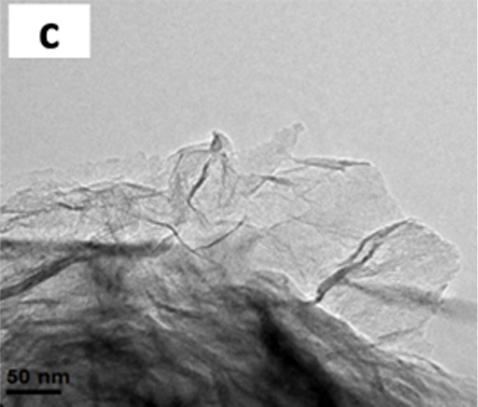
Figure 2. (a and b) Behavior of graphite and graphene oxide (GO) in water, c) GO sheet, Transmission Electron Microscopy (TEM).
The GO can be defined as a single exfoliated graphene sheet or stack of few sheets (3-4) that is functionalized with different oxygenated groups. Among its main characteristics is that it is hydrophilic, insulating and hygroscopic (absorbs moisture). On the other hand, graphene oxide sheets possess a large surface area and exhibit high mechanical strength and flexibility.
Applications
Graphene oxide has attracted great interest in various fields of science and technology, due to its remarkable mechanical, chemical, and thermal properties, among others. So numerous investigations began, to take advantage of the properties of graphene oxide.
In 2011, the first investigations of the use of GO as a precursor in the large-scale production of graphene emerged, for use as filler/reinforcement material/in polymeric matrices, such as high-density polyethylene (HDPE) and low-density polyethylene (HDPE). density (LDPE).
By 2014, GO was considered feasible for use as a flame retardant agent. Research is still ongoing to functionalize it with different polymeric materials.
In 2017, the first reports of the manufacture of GO-based membranes began, since it is impermeable to gases and liquids, showing its ability to filter small particles, organic molecules and even its use for seawater desalination.
In 2018, Energeia-Graphenemex started research on graphene oxide as a new additive for the production of anticorrosive and antimicrobial coatings. By 2019, studies of graphene oxide in coatings with antibacterial behavior increased, associated with the fact that GO is capable of penetrating the cell membrane of bacteria, producing oxidative stress and inhibiting their reproduction.
In particular, the functionalization of GO allows it to be applicable in biological systems, development of biosensors for the identification of specific molecules, drug delivery systems, among others.
Energeia – Graphenemex®, a leading Mexican company in Latin America in research and production of graphene materials for the development of industrial applications. It has extensive experience in the production of graphene oxide (GO) on a large scale, with different degrees of oxidation and high quality for use in different applications and industries. Currently, it uses graphene oxide in the production of concrete additives and anticorrosive and antimicrobial coatings that are marketed under the Graphenergy brand.
References
- M. Fang, K. Wang, H. Lu, Y. Yang y S. Nutt, «Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites,» Journal of Materials Chemistry, vol. 19, pp. 7098-7105, 2009.
- B. Dittrich, K.-a. Wartig, R. Mülhaupt y B. Schartel, «Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene,» Polymers, vol. 6, pp. 2875-2895, 2014.
- Y.-j. Wan, L.-x. Gong, L.-c. Tang, L.-b. Wu y J.-x. Jiang, «Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide,» COMPOSITES PART A, vol. 64, pp. 79-89, 2014.
- J. Wang, C. Xu, H. Hu, L. Wan, R. Chen, H. Zheng, F. Liu, M. Zhang, X. Shang y X. Wang, «Synthesis , mechanical , and barrier properties of LDPE / Graphene nanocomposites using vinyl triethoxysilane as a coupling agent,» J. Nanopart Res, vol. 13, pp. 869-878, 2011.

