Improve safety with flame retardant polymeric compounds
with graphene oxide
Polymeric compounds (engineering plastics) are widely used in the automotive, construction, food, aerospace and other sectors. Its use is based on the weight/resistance ratio, physical stability, chemical resistance and corrosion resistance.
However, most polymers, due to their nature, are flammable and combustible. That is, they are materials that catch fire quickly when exposed to fire, undergoing degradation, Veo complicadoand releasing heat to later start the propagation of the flames. During the combustion of polymers, they release smoke (soot) and toxic gases that are a danger to the safety of human life and property.
Four key components are involved during the combustion of polymeric materials: heat, oxygen, fuel, and free radical reaction. Flame retardancy of polymeric composites can be achieved by inhibiting or perturbing one or more of these components.
In recent years, multiple investigations have been carried out to develop additives that help inhibit or reduce the flammability of polymers, these additives are known as flame retardants.
Conventional flame retardants can be classified into two main categories, based on their components: inorganic flame retardants and organic flame retardants. The first include hydroxide, metal oxide, phosphate, silicate among others. They have excellent thermal stability, are non-toxic, are low cost and do not produce pollution. However, inorganic flame retardants are limited by high loading, low compatibility, and aggregation. On the other hand, organic flame retardants include flame retardants containing halogens, phosphorous, phosphorous-nitrogen, etc. The latter have high efficiency and good compatibility with polymers. Their main disadvantage is that they are restricted because they can release toxic gases and be harmful during combustion, endangering the health of people and the environment.

Graphene oxide (GO) is currently the most novel nanomaterial for use as a flame retardant because it exhibits high efficiency as a retardant with low loads and is non-toxic. Its efficiency is associated with the fact that graphene oxide has a strong barrier effect, high thermal stability and great surface absorption capacity, which are favorable for reducing heat and mass transfer.
Graphene-based flame retardants can improve the flame resistance of polymers by inhibiting the two key terms: heat and fuel. More specifically, graphene oxide can function as a flame retardant in different synergistic ways.
- First of all, GO has a unique two-dimensional layer structure and can promote the formation of a continuous dense layer of carbon during the combustion process. Carbon can act as a physical barrier to prevent heat transfer from the heat source and delay the escape of products (pyrolysis) from the polymeric substrate.
- Second, GO has a large specific surface area and can effectively adsorb flammable volatile organic compounds or hinder their release and diffusion during combustion.
- Third, GO contains abundant reactive oxygen-containing groups (carboxyl group at the edges, as well as epoxy and hydroxyl groups at the basal planes in the sheets). For example, oxygen-containing groups can undergo decomposition and dehydration at low temperatures, thus absorbing heat and cooling the polymeric substrate during combustion. Meanwhile, the gases generated by dehydration can dilute the oxygen concentration around the ignition periphery, decreasing the risk of fire spread.
- It can also modify the rheological behavior of the polymer and prevent its dripping, thus hindering the release and diffusion of volatile decomposition products through the ”maze effect” and affecting the flame retardancy of compounds (for example, modifying the UL-94 classification, oxygen index (OI) and time to ignition (TTI).

In studies carried out, it has been found that the incorporation of functionalized graphene oxide (5% by weight) in Polypropylene (PP) increased the Young’s modulus and the elastic limit of PP by 53% and 11%, respectively. While in the results of the flammability test (UL-94), it indicates that the presence of GO produces a change in the behavior of the melt and prevents the material from dripping.
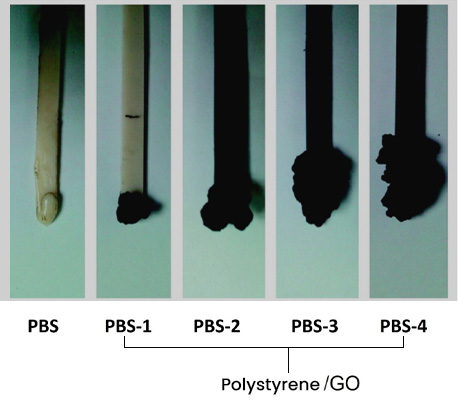
On the other hand, the preparation of polymeric compounds in melt blending (extrusion) of Polystyrene/GO have been reported, where it was found that GO (5%) can promote carbonization on the polymer surface (layer of carbonized material). and inside, the presence of a load or filler that presents high resistance to heat and contributes to the formation of carbon residues, improving the flame resistance of polystyrene-based compounds.
Currently Energeia – Graphenemex®, a leading Mexican company in Latin America in research and production of graphene materials for the development of applications at an industrial level, through its Graphenergy Masterbatch line, has developed a wide range of masterbatches with graphene oxide, based on various polymers, such as PP, HDPE, LDPE, PET and PA6.
The incorporation of graphene and graphene derivatives (GO) to polymeric matrices has allowed the development of polymeric compounds with better mechanical properties, greater thermal stability, gas barrier capacity and reduced flammability of polymeric compounds.
References
- Han Y, Wu Y, Shen M, Huang X, Zhu J, Zhang X. Preparation and properties of polystyrene nanocomposites with graphite oxide and graphene as flame retardants. J Mater Sci 48:4214.
- Hofmann D, Wartig K-A, Thomann R, Dittrich B, Schartel B, Mu¨lhaupt R. Functionalized graphene and carbon materials as additives for melt-extruded flame retardant polypropylene. Macromol Mater Eng 298:1322.
- Dittrich B, Wartig K-A, Hofmann D, Mu¨lhaupt R, Schartel B. Flame retardancy through carbon nanomaterials: carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym Degrad Stab 98:1495.

